Science and scientific data form the foundation for our statutory tasks at the MEB and the scientific analysis of data is actually an important basis for our assessments of medicines. The process of assessing medicines often leads to research questions which are not restricted to a single dossier and instead affect the entire regulatory system. We look for answers to these questions by conducting scientific research in the domain of ‘Regulatory Science’.
Regulatory Science is an applied science which, via various scientific disciplines, assesses internal regulations and policy in relation to the assessment of the entire life cycle of medicines. New insights contribute to ‘evidence based regulatory practice’: It involves answering questions such as: are we doing things properly, do adjustments need to be made on the basis of new knowledge and are we prepared, based on our current knowledge and expertise, for change and innovation?’ Regulatory Science is also aimed at the development and improvement of instruments, standards and working methods used to assess medicines in terms of efficacy, risks and quality and the improvement and innovation of the system as a whole.
System improvement and innovation
The MEB's scientific program focuses on system improvement and innovation: optimizing our current work processes, deregulation of our regulatory system through constant review and responding to developments with added value for the MEB's role and task. These scientific activities serve several purposes:
- To continue to ensure the availability and accessibility of medicines for patients by having the latest scientific insights, innovations, tools, and expertise for the high-quality assessment of medicines.
- To innovate and improve our regulatory system through continuous evaluation of existing regulations and by influencing international guidelines and policies.
- To safeguard the future-readiness of the organisation by anticipating and contributing to new and innovative developments.
- To embed and secure knowledge in our work by translating scientific insights and results into daily (assessment) practice.
- To inspire and support the development of (potential) staff by enabling them to combine research, supervisory, or teaching activities with assessment work.
- To combine our internal expertise with the knowledge and capabilities of academic groups and other knowledge institutions by contributing to a strong (inter)national scientific and regulatory network.
Cooperation
Regulatory Science is a cross-border phenomenon and extends across the entire international regulatory system. That is why, when carrying out scientific activities, we frequently collaborate with academic groups, other scientific knowledge institutions and other authorities in our network (national medicines authorities and the European Medicines Agency).
We are also a member of various public-private partnerships, such as the Regulatory Science Network Netherlands (RSNN), Horizon Europe and Innovative Medicine Initiative (IMI) projects, in which patient representatives and pharmaceutical umbrella organisations participate. Thanks to this continuous cooperation with, and knowledge sharing and exchanges within our network, we are able to implement solutions which make the process of regulating medicines faster, more efficient and more flexible.
In addition, the MEB organises the MEB Science Day every year to bring together representatives from medicine authorities, healthcare, the world of academia and industry.
Regulatory Science: Magazine and LinkedIn
Between 2017 and 2022 the MEB has published at least two editions of the Regulatory Science Magazine each year to keep you updated on recent highlights of regulatory science. To share information more frequently, we started a showcase LinkedIn page at the end of 2022. Through this LinkedIn page we now keep you updated about regulatory science and recent publications, share interviews, and the latest information on scientific events.
Research
Various PhD students and other university students are performing research at the MEB. PhD students divide their time between their research and helping to assess medicine dossiers. This creates an excellent link between science and assessment in practice. The Science Committee decides which research the MEB should be involved in and which research projects are eligible for funding and also guarantees scientific quality during the various research procedures.
News
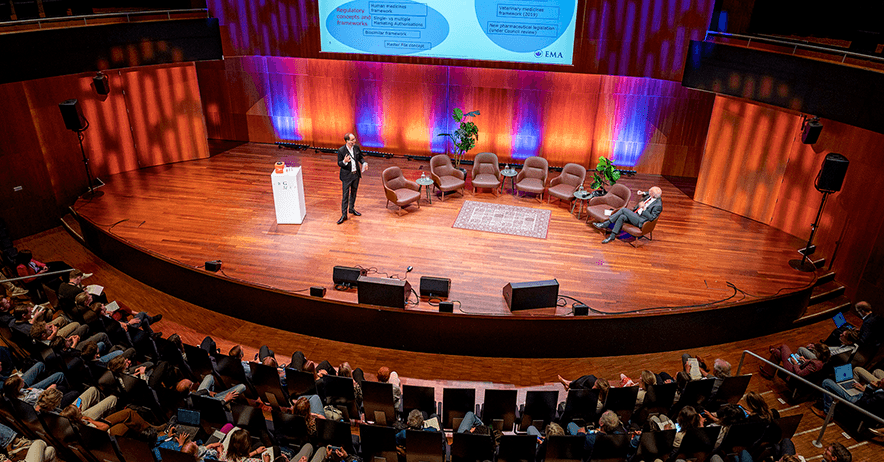
MEB Science Day 2025 – Platform Technologies: opportunities and challenges
Platform technologies have recently gained increased attention due to their potential to advance medicines development. A growing ...
Read more
Science Policy 2025–2029: From expertise to impact
The MEB has a new Science Policy for 2025-2029. Four key themes will guide regulatory science at the MEB for the coming four ...
Read more
MEB Science Day 2025 - Platform Technologies: opportunities and challenges
Join us for the MEB Science Day 2025, a unique opportunity to share knowledge and connect with colleagues in the field of ...
Read more
MEB Science Day 2024: Human centric approaches to improve the success of oncology therapies
The MEB Science Day 2024 took place in the Princess Máxima Center for Pediatric Oncology in Utrecht on 16 April 2024 and focused ...
Read more
MEB Science Day 2023: Biomarkers and Companion Diagnostics
This year’s MEB Science Day focused on the future of precision medicine. The use of biomarkers and companion diagnostics plays an ...
Read more
STARS: ‘A matter of bringing worlds together’
June 2022 marked the finalisation of the three year period of STARS (Strengthening Training of Academia in Regulatory Science). ...
Read more