News
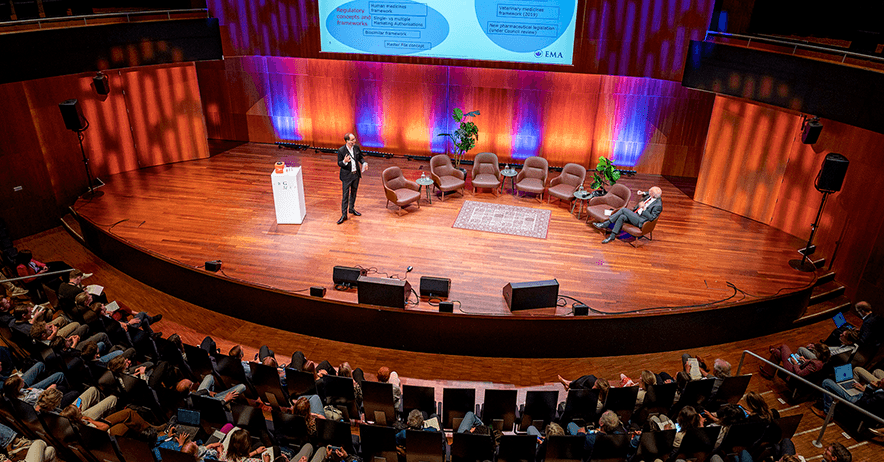
MEB Science Day 2025 – Platform Technologies: opportunities and challenges
Platform technologies have recently gained increased attention due to their potential to advance medicines development. A growing ...
Read more
Science Policy 2025–2029: From expertise to impact
The MEB has a new Science Policy for 2025-2029. Four key themes will guide regulatory science at the MEB for the coming four ...
Read more
MEB Science Day 2025 - Platform Technologies: opportunities and challenges
Join us for the MEB Science Day 2025, a unique opportunity to share knowledge and connect with colleagues in the field of ...
Read more
MEB Science Day 2024: Human centric approaches to improve the success of oncology therapies
The MEB Science Day 2024 took place in the Princess Máxima Center for Pediatric Oncology in Utrecht on 16 April 2024 and focused ...
Read more
First electronic product information (ePI) published
The Medicines Evaluation Board (MEB), in collaboration with 4 other medicines agencies, has published the first electronic ...
Read more
MEB starts pilot project to test electronic product information
The Medicines Evaluation Board (MEB) is starting a pilot project to generate an electronic format for medicinal product ...
Read more
