News

MEB fee rate change as of January 2026
The Medicines Evaluation Board (MEB) increases the fee rates for authorisation applications, authorisation changes and the annual ...
Read more
New Veterinary Medicinal Products Unit fees for 2026
With effect from 1 January 2026, the Medicines Evaluation Board (MEB) will be increasing its fees. This increase is necessary due ...
Read more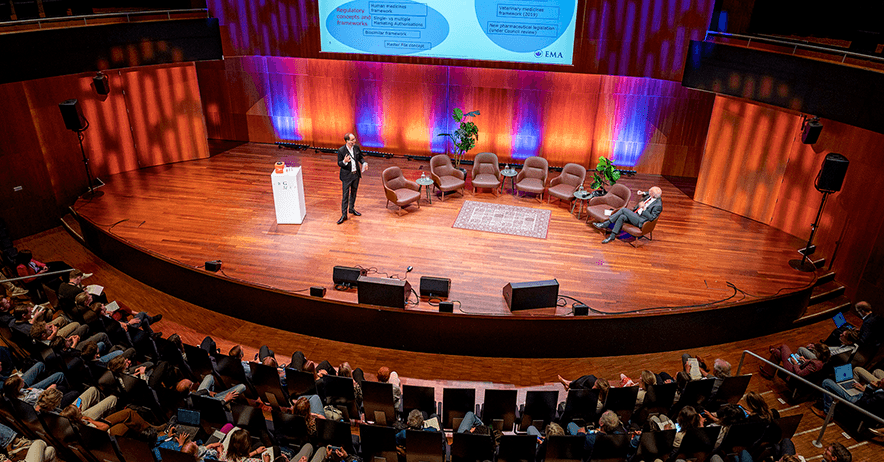
MEB Science Day 2025 – Platform Technologies: opportunities and challenges
Platform technologies have recently gained increased attention due to their potential to advance medicines development. A growing ...
Read more
The Veterinary Medicinal Product Unit publishes quarterly overview of companies in the starting materials (API) register.
As of July 1 2025, the Veterinary Medicinal Product Unit (BD) will publish quarterly overviews of manufacturers, importers, ...
Read more
New availability schedule of time slots
The moment at which new slots become available in the MEB planning tool- used by companies to reserve a timeslot for submitting ...
Read more
Advice on implementing regulation (EU) 2024/1159
The European Medicines Agency (EMA) published a new version of the QRD template in November 2024: QRD template version 9.1. ...
Read more
Science Policy 2025–2029: From expertise to impact
The MEB has a new Science Policy for 2025-2029. Four key themes will guide regulatory science at the MEB for the coming four ...
Read more
VMPU to start publication of GMP inspections
Starting this year, the Veterinary Medicinal Products Unit (VMPU) will publish the reports of veterinary GMP inspections. The ...
Read more
Planning tool for requesting time slots mandatory from 1 July 2025
As of 1 July 2025 it will be mandatory to use the MEB planning tool to reserve a time slot for submitting an application for ...
Read more
MEB Science Day 2025 - Platform Technologies: opportunities and challenges
Join us for the MEB Science Day 2025, a unique opportunity to share knowledge and connect with colleagues in the field of ...
Read more
New veterinary QRD template version 9.1 published
A Summary of Product Characteristics (SPC), a label and a package leaflet are established when a marketing authorisation is ...
Read more
European Shortages Monitoring Platform operational
The European Shortages Monitoring Platform (ESMP) went live this week. The platform enables marketing authorisation holders and ...
Read more
MEB fee rate change as of January 2025
The Medicines Evaluation Board (MEB) increases the fee rates for authorisation applications, authorisation changes and the annual ...
Read more
New Veterinary Medicinal Products Unit fees for 2025
With effect from 1 January 2025, the Medicines Evaluation Board (MEB) will be increasing its fees. This increase is necessary due ...
Read more
Revised notification form for medicine shortages and defects
The notification form of the Medicine Shortages and Defects Notification Centre has been made more user-friendly. The marketing ...
Read more
MEB Science Day 2024: Human centric approaches to improve the success of oncology therapies
The MEB Science Day 2024 took place in the Princess Máxima Center for Pediatric Oncology in Utrecht on 16 April 2024 and focused ...
Read more
Planning tool for requesting timeslots expanded to more procedures
The planning tool firms can use to reserve a timeslot for requesting assessment procedures is being expanded. From now on, the ...
Read more
MEB fee rate change as of January 2024
The Medicines Evaluation Board (MEB) increases the fee rates for authorisation applications, authorisation changes and the annual ...
Read more